XIX. Three Papers, Containing Experiments on Factitious Air, by the Hon. Henry Cavendish, F.R.S.
Read May 29, Nov. 6 and Nov. 13, 1766.
By factitious air, I mean in general any kind of air which is contained in other bodies in an unelastic state, and is produced from thence by art.
By fixed air, I mean that particular species of factitious air, which is separated from alcaline substances by solution in acids or by calcination; and to which Dr. Black has given that name in his treatise on quick lime.
As fixed air makes a considerable part of the subject of the following papers, and as the name might incline one to think, that it signified any sort of air which is contained in other bodies in an unelastic form; I thought it best to give this explanation before I went any farther. . . .
EXPERIMENTS ON FACTITIOUS AIR PART I CONTAINING EXPERIMENTS ON INFLAMMABLE AIR
I know of only three metallic substances, namely, zinc, iron and tin, that generate inflammable air by solution in acids; and those only by solution in the diluted vitriolic acid, or spirit of salt.
Zinc dissolves with great rapidity in both these acids; and, unless they are very much diluted, generates a considerable heat. One ounce of zinc produces about 356 ounce measures of air: the quantity seems just the same whichsoever of these acids it is dissolved in. Iron dissolves readily in the diluted vitriolic acid, but not near so readily as zinc. One ounce of iron wire produces about 412 ounce measures of air: the quantity was just the same, whether the oil of vitriol was diluted with 1½, or 7 times its weight of water: so that the quantity of air produced seems not at all to depend on the strength of acid.
Iron dissolves but slowly in spirit of salt while cold: with the assistance of heat it dissolves moderately fast. The air produced thereby is inflammable, but I have not tried how much it produces.
Tin was found to dissolve scarce at all in oil of vitriol diluted with an equal weight of water, while cold: with the assistance of a moderate heat it dissolved slowly, and generated air, which was inflammable: the quantity was not ascertained.
Tin dissolves slowly in strong spirit of salt while cold: with the assistance of heat it dissolves moderately fast. One ounce of tinfoil yields 202 ounce measures of inflammable air.
These experiments were made, when the thermometer was at 50° and the barometer at 30 inches.
All these three metallic substances dissolve readily in the nitrous acid, and generate air; but the air is not at all inflammable. They also unite readily, with the assistance of heat, to the undiluted acid of vitriol; but very little of the salt, formed by their union with the acid, dissolves in the fluid. They all unite to the acid with a considerable effervescence, and discharge plenty of vapours, which smell strongly of the volatile sulphureous acid, and which are not at all inflammable. Iron is not sensibly acted on by this acid, without the assistance of heat; but zinc and tin are in some measure acted on by it, while cold.
It seems likely from hence, that, when either of the above-mentioned metallic substances are dissolved in spirit of salt, or the diluted vitriolic acid, their phlogiston flies off, without having its nature changed by the acid, and forms the inflammable air; but that, when they are dissolved in the nitrous acid, or united by heat to the vitriolic acid, their phlogiston unites to part of the acid used for their solution, and flies off with it in fumes, the phlogiston losing its inflammable property by the union. The volatile sulphureous fumes, produced by uniting these metallic substances by heat to the undiluted vitriolic acid, shew plainly, that in this case their phlogiston unites to the acid; for it is well known, that the vitriolic sulphureous acid consists of the plain vitriolic acid united to phlogiston.4 It is highly probable too, that the same thing happens in dissolving these metallic substances in the nitrous acid; as the fumes produced during the solution appear plainly to consist in great measure of the nitrous acid, and yet it appears, from their more penetrating smell and other reasons, that the acid must have undergone some change in its nature, which can hardly be attributed to anything else than its union with the phlogiston. As to the inflammable air, produced by dissolving these substances in spirit of salt or the diluted vitriolic acid, there is great reason to think, that it does not contain any of the acid in its composition; not only because it seems to be just the same whichsoever of these acids it is produced by; but also because there is an inflammable air, seemingly much of the same kind as this, produced from animal substances in putrefaction, and from vegetable substances in distillation, as will be shewen hereafter; though there can be no reason to suppose, that this kind of inflammable air owes its production to any acid. I now proceed to the experiments made on inflammable air.
I cannot find that this air has any tendency to lose its elasticity by keeping, or that it is all absorbed, either by water, or by fixed or volatile alcalies; as I have kept some by me for several weeks in a bottle inverted into a vessel of water, without any sensible decrease of bulk; and as I have also kept some for a few days, in bottles inverted into vessels of sope leys and spirit of sal ammoniac, without perceiving their bulk to be at all diminished.
It has been observed by others, that, when a piece of lighted paper is applied to the mouth of a bottle, containing a mixture of inflammable and common air, the air takes fire, and goes off with an explosion. In order to observe in what manner the effect varies according to the different proportions in which they are mixed, the following experiment was made.
Some of the inflammable air, produced by dissolving zinc in diluted oil of vitriol, was mixed with common air in several different proportions, and the inflammability of these mixtures tried one after the other in this manner. A quart bottle was filled with one of these mixtures. . . . The bottle was taken out of the water, set upright on a table, and the flame of a lamp or piece of lighted paper applied to its mouth. But, in order to prevent the included air from mixing with the outward air, before the flame could be applied, the mouth of the bottle was covered while under water, with a cap made of a piece of wood covered with a few folds of linnen; which cap was not removed till the instant that the flame was applied. The mixtures were all tried in the same bottle; and, as they were all ready prepared, before the inflammability of any of them was tried, the time elapsed between each trial was but small: by which means I was better able to compare the loudness of the sound in each trial. The result of the experiment is as follows.
With one part of inflammable air to 9 of common air, the mixture would not take fire, on applying the lighted paper to the mouth of the bottle; but, on putting it down into the belly of the bottle, the air took fire, but made very little sound.
With 2 parts of inflammable to 8 of common air, it took fire immediately, on applying the flame to the mouth of the bottle, and went off with a moderately loud noise.
With 3 parts of inflammable air to 7 of common air, there was a very loud noise.
With 4 parts of inflammable to 6 of common air, the sound seemed very little louder.
With equal quantities of inflammable and common air, the sound seemed much the same. In the first of these trials, namely, that with one part of inflammable to 9 of common air, the mixture did not take fire all at once, on putting the lighted paper into the bottle; but one might perceive the flame to spread gradually through the bottle. In the three next trials, though they made an explosion, yet I could not perceive any light within the bottle. In all probability, the flame spread so instantly through the bottle, and was so soon over, that it had not time to make any impression on my eye. In the last mentioned trial, namely, that with equal quantities of inflammable and common air, a light was seen in the bottle, but which quickly ceased.
With 6 parts of inflammable to 4 of common air, the sound was not very loud: the mixture continued burning a short time in the bottle, after the sound was over.
With 7 parts of inflammable to 3 of common air, there was a very gentle bounce or rather puff: it continued burning for some seconds in the belly of the bottle.
A mixture of 8 parts of inflammable to 2 of common air caught fire on applying the flame, but without any noise: it continued burning for some time in the neck of the bottle, and then went out, without the flame ever extending into the belly of the bottle.
It appears from these experiments, that this air, like other inflammable substances, cannot burn without the assistance of common air. It seems too, that, unless the mixture contains more common than inflammable air, the common air therein is not sufficient to consume the whole of the inflammable air; whereby part of the inflammable air remains, and burns by means of the common air, which rushes into the bottle after the explosion.
In order to find whether there was any difference in point of inflammability between the air produced from different metals by different acids, five different sorts of air, namely, 1. Some produced from zinc by diluted oil of vitriol, and which had been kept about a fortnight; 2. Some of the same kind of air fresh made; 3. Air produced from zinc by spirit of salt; 4. Air from iron by the vitriolic acid; 5. Air from tin by spirit of salt; were each mixed separately with common air in the proportion of 2 parts of inflammable air to
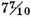
of common air, and their inflammability tried in the same bottle, that was used for the former experiment, and with the same precautions. They each went off with a pretty loud noise, and without any difference in the sound that I could be sure of. Some more of each of the above parcels of air were then mixed with common air, in the proportion of 7 parts of inflammable air to
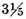
of common air, and tried in the same way as before. They each of them went off with a gentle bounce, and burnt some time in the bottle, without my being able to perceive any difference between them.
In order to avoid being hurt, in ease the bottle should burst by the explosion, I have commonly, in making these sort of experiments, made use of an apparatus contrived in such manner, that, by pulling a string, I drew the flame of a lamp over the mouth of the bottle, and at the same time pulled off the cap, while I stood out of the reach of danger. I believe, however, that this precaution is not very necessary; as I have never known a bottle to burst in any of the trials I have made.
The specific gravity of each of the above-mentioned sorts of inflammable air, except the first, was tried in the following manner. A bladder holding about 100 ounce measures was filled with inflammable air, . . . and the air pressed out again as perfectly as possible. By this means the small quantity of air remaining in the bladder was almost intirely of the inflammable kind. 80 ounce measures of the inflammable air, produced from zinc by the vitriolic acid, were then forced into the bladder in the same manner: after which, the pewter pipe was taken out of the wooden cap of the bladder, the orifice of the cap stopt up with a bit of lute, and the bladder weighed. A hole was then made in the lute, the air pressed out as perfectly as possible, and the bladder weighed again. It was found to have increased in weight 40¾ grains. Therefore the air pressed out of the bladder weighs 40¾ grains less than an equal quantity of common air: but the quantity of air pressed out of the bladder must be nearly the same as that which was forced into it, i.e. 80 ounce measures: consequently 80
ounce measures of this sort of inflammable air weigh 40¾ grains less than an equal bulk of common air. The three other sorts of inflammable air were then tried in the same way, in the same bladder, immediately one after the other. In the trial with air from zinc by spirit of salt, the bladder increased 40½ grains on forcing out the air. In the trial with the air from iron, it increased 41½ grains, and in that with the air from tin, it increased 41 grains. The heat of the air, when this experiment was made, was 50°; the barometer stood at 29¾ inches.
There seems no reason to imagine, from these experiments, that there is any difference in point of specific gravity between these four sorts of inflammable air; as the small difference observed in these trials is in all probability less than what may arise from the unavoidable errors of the experiment. Taking a medium therefore of the different trials, 80 ounce measures of inflammable air weigh 41 grains less than an equal bulk of common air.
Therefore, if the density of common air, at the time when this experiment was tried, was 800 times less than that of water, which, I imagine, must be near the truth, inflammable air must be 5490 times lighter than water, or near 7 times lighter than common air. But if the density of common air was 850 times less than that of water, then would inflammable air be 9200 times lighter than water, or
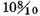
lighter than common air.
This method of finding the density of factitious air is very convenient and sufficiently accurate, where the density of the air to be tried is not much less than that of common air, but cannot be much depended on in the present case, both on account of the uncertainty in the density of common air, and because we cannot be certain but what some common air might be mixed with the inflammable air in the bladder, notwithstanding the precautions used to prevent it; both which causes may produce a considerable error, where the density of the air to be tried is many times less than that of common air. For this reason, I made the following experiments.
I endeavoured to find the weight of the air discharged from a given quantity of zinc by solution in the vitriolic acid. . . . A is a bottle filled near full with oil of vitriol diluted with about six times its weight of water: B is a glass tube fitted into its mouth, and secured with lute: C is a glass cylinder fastened on the end of the tube, and secured also with lute. The cylinder has a small hole at its upper end to let the inflammable air escape, and is filled with dry pearl-ashes in coarse powder. The whole apparatus, together with the zinc, which was intended to be put in, and the lute which was to be used in securing the tube to the neck of the bottle, were first weighed carefully; its weight was 11930 grains. The zinc was then put in, and the tube put in its place. By this means, the inflammable air was made to pass through the dry pearl-ashes; whereby it must have been pretty effectually deprived of any acid or watery vapours that could have ascended along with it. The use of the glass tube B was to collect the minute jets of liquor, that were thrown up by the effervescence, and to prevent their touching the pearl-ashes; for which reason, a small space was left between the glass-tube and the pearl-ashes in the cylinder. When the zinc was dissolved, the whole apparatus was weighed again, and was found to have lost 11¾ grains in weight;5 which loss is principally owing to the weight of the inflammable air discharged. But it must be observed, that, before the effervescence, that part of the bottle and cylinder, which was not occupied by other more solid matter, was filled with common air; whereas, after the effervescence, it was filled with inflammable air; so that, upon that account alone, supposing no more inflammable air to be discharged than what was sufficient to fill that space, the weight of the apparatus would have been diminished by the difference of the weight of that quantity of common air and inflammable air. The whole empty space in the bottle and cylinder was about 980 grain measures, there is no need of exactness; and the difference of the weight of that quantity of common and inflammable air is about one grain: therefore the true weight of the inflammable air discharged, is 10¾ grains. The quantity of zinc used was 254 grains, and consequently the weight of the air discharged is
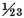
or
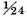
of the weight of the zinc.
It was before said, that one grain of zinc yielded 356 grain measures of air: therefore 254 grains of zinc yield 90427 grain measures of air; which we have just found to weigh 10¾ grains; therefore inflammable air is about 8410 times lighter than water, or 10½ times lighter than common air.
The quantity of moisture condensed in the pearl-ashes was found to be about 1¼ grains.
By another experiment, tried exactly in the same way, the density of inflammable air came out 8300 times less than that of water.
The specific gravity of the air, produced by dissolving zinc in spirit of salt, was tried exactly in the same manner. 244 grains of zinc being dissolved in spirit of salt diluted with about four times its weight of water, the loss in effervescence was 10¾ grains; the empty space in the bottle and cylinder was 914 grain measures; whence the weight of the inflammable air was 9¾ grains, and consequently its density was 8910 times less than that of water.
By another experiment, its specific gravity came out 9030 times lighter than water.
A like experiment was tried with iron. 250½ grains of iron being dissolved in oil of vitriol diluted with four times its weight of water, the loss in effervescence was 13 grains, the empty space 1420 grain measures. Therefore the weight of the inflammable air was
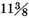
grains, i.e. about
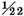
of the weight of the iron, and its density was 8973 times less than that of water. The moisture condensed was 1¼ grains.
A like experiment was tried with tin. 607 grains of tinfoil being dissolved in strong spirit of salt, the loss in effervescence was 14¾ grains, the empty space 873 grain measures: therefore the weight of the inflammable air was 13¾ grains, i.e.
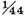
of the tin, and its density 8918 times less than that of water. The quantity of moisture condensed was about three grains.
It is evident, that the truth of these determinations depend[s] on a supposition, that none of the inflammable air is absorbed by the pearl-ashes. In order to see whether this was the case or no, I dissolved 86 grains of zinc in diluted acid of vitriol, and received the air in a measuring bottle in the common way. Immediately after, I dissolved the same quantity of zinc in the same kind of acid, and made the air to pass into the same measuring bottle, through a cylinder filled with dry pearl-ashes. . . . I could not perceive any difference in their bulks.
It appears from these experiments, that there is but little, if any, difference in point of density between the different sorts of inflammable air. Whether the difference of density observed between the air procured from zinc, by the vitriolic and that by the marine acid is real, or whether it is only owing to the error of the experiment, I cannot pretend to say. By a medium of the experiments, inflammable air comes out 8760 times lighter than water, or eleven times lighter than common air.
In order to see whether inflammable air, in the state in which it is, when contained in the inverted bottles, where it is in contact with water, contains any considerable quantity of moisture dissolved in it, I forced 192 ounce measures of inflammable air, through a cylinder filled with dry pearl-ashes, by means of the same apparatus, which I used for filling the bladders with inflammable air. . . . The cylinder was weighed carefully before and after the air was forced through; whereby it was found to have increased 1 grain in weight. The empty space in the cylinder was 248 grains, the difference of weight of which quantity of common and inflammable air is ¼ of a grain. Therefore the real quantity of moisture condensed in the pearl-ashes is 1¼ grain. The weight of 192 ounce measures of inflammable air deprived of its moisture appears from the former experiments to be 10½ grains; therefore its weight when saturated with moisture would be 11¾ grains. Therefore, inflammable air, in that state in which it is in, when kept under the inverted bottles, contains near
its weight of moisture; and its specific gravity in that state is 7840 times less than that of water.
XIII. EXPERIMENTS ON AIR. BY HENRY CAVENDISH, ESQ, F.R.S. & S.A.
Read January 15, 1784.
The following experiments were made principally with a view to find out the cause of the diminution which common air is well known to suffer by all the various ways in which it is phlogisticated, and to discover what becomes of the air thus lost or condensed; and as they seem not only to determine this point, but also to throw great light on the constitution and manner of production of dephlogisticated air, I hope they may be not unworthy the acceptance of this society.
Many gentlemen have supposed that fixed air is either generated or separated from atmospheric air by phlogistication, and that the observed diminution is owing to this cause; my first experiments therefore were made in order to ascertain whether any fixed air is really produced thereby.
Cavendish here rejects animal and vegetable substances as fit subjects for these experiments, since they are known to yield fixed air of themselves, while he wishes to show that fixed air is not produced from common air itself in its "diminution." Thereafter. he cites experiments to show that common air will not yield fixed air in the calcination of metals, in the burning of sulfur, of phosphorus, or inflammable air. He also shows that neither vitriolic (sulfuric)or nitrous (nitric) acids are formed from the air itself. He goes on to say,
Having now mentioned the unsuccessful attempts I made to find out what becomes of the air lost by phlogistication, I proceed to some experiments, which serve really to explain the matter.
In Dr. PRIESTLEY’s last volume of experiments is related an experiment of Mr. WARLTIRE’s, in which it is said that, on firing a mixture of common and inflammable air by electricity in a close copper vessel holding about three pints, a loss of weight was always perceived, on an average about two grains, though the vessel was stopped in such a manner that no air could escape by the explosion. It is also related, that on repeating the experiment in glass vessels, the inside of the glass, though clean and dry before, immediately became dewy; which confirmed an opinion he had long entertained, that common air deposits its moisture by phlogistication. As the latter experiment seemed likely to throw great light on the subject I had in view, I thought it well worth examining more closely. The first experiment also, if there was no mistake in it, would be very extraordinary and curious; but it did not succeed with me; for though the vessel I used held more than Mr. WARLTIRE’s, namely, 24,000 grains of water, and though the experiment was repeated several times with different proportions of common and inflammable air, I could never perceive a loss of weight of more than one-fifth of a grain, and commonly none at all. It must be observed, however, that though there were some of the experiments in which it seemed to diminish a little in weight, there were none in which it increased.9
In all the experiments, the inside of the glass globe became dewy, as observed by Mr. WARLTIRE; but not the least sooty matter could be perceived. Care was taken in all of them to find how much the air was diminished by the explosion, and to observe its test. The result is as follows: the bulk of the inflammable air being expressed in decimals of the common air.
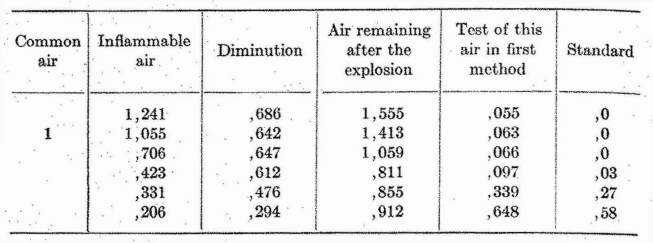
In these experiments the inflammable air was procured from zinc, as it was in all my experiments, except where otherwise expressed: but I made two more experiments, to try whether there was any difference between the air from zinc and that from iron, the quantity of inflammable air being the same in both, namely, 0,331 of the common; but I could not find any difference to be depended on between the two kinds of air, either in the diminution which they suffered by the explosion, or the test of the burnt air.
From the fourth experiment it appears, that 423 measures of inflammable air are nearly sufficient to completely phlogisticate 1000 of common air; and that the bulk of the air remaining after the explosion is then very little more than four-fifths of the common air employed; so that as common air cannot be reduced to a much less bulk than that by any method of phlogistication, we may safely conclude, that when they are mixed in this proportion, and exploded, almost all the inflammable air, and about one-fifth part of the common air, lose their elasticity, and are condensed into the dew which lines the glass.
The better to examine the nature of this dew, 500,000 grain measures of inflammable air were burnt with about 2½ times that quantity of common air, and the burnt air made to pass through a glass cylinder eight feet long and three-quarters of an inch in diameter, in order to deposit the dew. The two airs were conveyed slowly into this cylinder by separate copper pipes, passing through a brass plate which stopped up the end of the cylinder; and as neither inflammable nor common air can burn by themselves, there was no danger of the flame spreading into the magazines from which they were conveyed. Each of these magazines consisted of a large tin vessel, inverted into another vessel just big enough to receive it. The inner vessel communicated with the copper pipe, and the air was forced out of it by pouring water into the outer vessel; and in order that the quantity of common air expelled should be 2½ times that of the inflammable, the water was let into the outer vessels by two holes in the bottom of the same tin pan, the hole which conveyed the water into that vessel in which the common air was confined being 2½ times as big as the other.
In trying the experiment, the magazines being first filled with their respective airs, the glass cylinder was taken off, and water let, by the two holes, into the outer vessels, till the airs began to issue from the ends of the copper pipes; they were then set on fire by a candle, and the cylinder put on again in its place. By this means upwards of 135 grains of water were condensed in the cylinder, which had no taste nor smell, and which left no sensible sediment when evaporated to dryness; neither did it yield any pungent smell during the evaporation; in short, it seemed pure water.
In my first experiment, the cylinder near that part where the air was fired was a little tinged with sooty matter, but very slightly so; and that little seemed to proceed from the putty with which the apparatus was luted, and which was heated by the flame; for in another experiment, in which it was contrived so that the luting should not be much heated, scarce any sooty tinge could be perceived.
By the experiments with the globe it appeared, that when inflammable and common air are exploded in a proper proportion, almost all the inflammable air, and near one-fifth of the common air, lose their elasticity, and are condensed into dew. And by this experiment it appears, that this dew is plain water, and consequently that almost all the inflammable air, and about one-fifth of the common air, are turned into pure water.
In order to examine the nature of the matter condensed on firing a mixture of dephlogisticated and inflammable air, I took a glass globe, holding 8800 grain measures, furnished with a brass cock and an apparatus for firing air by electricity. This globe was well exhausted by an air-pump, and then filled with a mixture of inflammable and dephlogisticated air, by shutting the cock, fastening a bent glass tube to its mouth, and letting up the end of it into a glass jar inverted into water, and containing a mixture of 19,500 grain measures of dephlogisticated air, and 37,000 of inflammable; so that, upon opening the cock, some of this mixed air rushed through the bent tube, and filled the globe.10 The cock was then shut, and the included air fired by electricity, by which means almost all of it lost its elasticity. The cock was then again opened, so as to let in more of the same air, to supply the place of that destroyed by the explosion, which was again fired, and the operation continued till almost the whole of the mixture was let into the globe and exploded. By this means, though the globe held not more than the sixth part of the mixture, almost the whole of it was exploded therein, without any fresh exhaustion of the globe.
As I was desirous to try the quantity and test of this burnt air, without letting any water into the globe, which would have prevented my examining the nature of the condensed matter, I took a larger globe, furnished also with a stop cock, exhausted it by an air-pump, and screwed it on upon the cock of the former globe; upon which, by opening both cocks, the air rushed out of the smaller globe into the larger, till it became of equal density in both; then, by shutting the cock of the larger globe, unscrewing it again from the former, and opening it under water, I was enabled to find the quantity of burnt air in it; and consequently, as the proportion which the contents of the two globes bore to each other was known, could tell the quantity of burnt air in the small globe before the communication was made between them. By this means the whole quantity of the burnt air was found to be 2950 grain measures; its standard was 1,85.
The liquor condensed in the globe, in weight about 30 grains, was sensibly acid to the taste, and by saturation with fixed alkali, and evaporation, yielded near two grains of nitre; so that it consisted of water united to a small quantity of nitrous acid. No sooty matter was deposited in the globe. The dephlogisticated air used in this experiment was procured from red precipitate, that is, from a solution of quicksilver in spirit of nitre distilled till it acquires a red colour.
As it was suspected, that the acid contained in the condensed liquor was no essential part of the dephlogisticated air, but was owing to some acid vapour which came over in making it and had not been absorbed by the water, the experiment was repeated in the same manner, with some more of the same air, which had been previously washed with water, by keeping it a day or two in a bottle with some water, and shaking it frequently; whereas that used in the preceding experiment had never passed through water, except in preparing it. The condensed liquor was still acid.
The experiment was also repeated with dephlogisticated air, procured from red lead by means of oil of vitriol; the liquor condensed was acid, but by an accident I was prevented from determining the nature of the acid.
I also procured some dephlogisticated air from the leaves of plants, in the manner of Doctors INGENHOUSZ and PRIESTLEY, and exploded it with inflammable air as before; the condensed liquor still continued acid, and of the nitrous kind.
In all these experiments the proportion of inflammable air was such, that the burnt air was not much phlogisticated, and it was observed, that the less phlogisticated it was, the more acid was the condensed liquor. I therefore made another experiment, with some more of the same air from plants, in which the proportion of inflammable air was greater, so that the burnt air was almost completely phlogisticated, its standard being
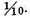
The condensed liquor was then not at all acid, but seemed pure water: so that it appears, that with this kind of dephlogisticated air, the condensed liquor is not at all acid, when the two airs are mixed in such a proportion that the burnt air is almost completely phlogisticated, but is considerably so when it is not much phlogisticated.
In order to see whether the same thing would obtain with air procured from red precipitate, I made two more experiments with that kind of air, the air in both being taken from the same bottle, and the experiment tried in the same manner, except that the proportions of inflammable air were different. In the first, in which the burnt air was almost completely phlogisticated, the condensed liquor was not at all acid. In the second, in which its standard was 1,86, that is, not much phlogisticated, it was considerably acid; so that with this air, as well as with that from plants, the condensed liquor contains, or is entirely free from, acid, according as the burnt air is less or more phlogisticated; and there can be little doubt but that the same rule obtains with any other kind of dephlogisticated air.
In order to see whether the acid, formed by the explosion of dephlogisticated air obtained by means of the vitriolic acid, would also be of the nitrous kind, I procured some air from turbith mineral [basic mercury sulfate], and exploded it with inflammable air, the proportion being such that the burnt air was not much phlogisticated. The condensed liquor manifested an acidity, which appeared, by saturation with a solution of salt of tartar [potassium carbonate], to be of the nitrous kind; and it was found, by the addition of some terra ponderosa salita [barium chloride], to contain little or no vitriolic acid.
When inflammable air was exploded with common air, in such a proportion that the standard of the burnt air was about
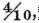
the condensed liquor was not in the least acid. There is no difference, however, in this respect between common air, and dephlogisticated air mixed with phlogisticated in such a proportion as to reduce it to the standard of common air; for some dephlogisticated air from red precipitate, being reduced to this standard by the addition of perfectly phlogisticated air, and then exploded with the same proportion of inflammable air as the common air was in the foregoing experiment, the condensed liquor was not in the least acid.
From the foregoing experiments it appears, that when a mixture of inflammable and dephlogisticated air is exploded in such proportion that the burnt air is not much phlogisticated, the condensed liquor contains a little acid, which is always of the nitrous kind, whatever substance the dephlogisticated air is procured from: but if the proportion be such that the burnt air is almost entirely phlogisticated, the condensed liquor is not at all acid, but seems pure water, without any addition whatever; and as, when they are mixed in that proportion, very little air remains after the explosion, almost the whole being condensed, it follows, that almost the whole of the inflammable and dephlogisticated air is converted into pure water. It is not easy, indeed, to determine from these experiments what proportion the burnt air, remaining after the explosions, bore to the dephlogisticated air employed, as neither the small nor the large globe could be perfectly exhausted of air, and there was no saying with exactness what quantity was left in them; but in most of them, after allowing for this uncertainty, the true quantity of burnt air seemed not more than
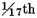
of the dephlogisticated air employed, or
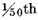
of the mixture. It seems, however, unnecessary to determine this point exactly, as the quantity is so small, that there can be little doubt but that it proceeds only from the impurities mixed with the dephlogisticated and inflammable air, and consequently that, if those airs could be obtained perfectly pure, the whole would be condensed.
With respect to common air, and dephlogisticated air reduced by the addition of phlogistieated air to the standard of common air, the case is different; as the liquor condensed in exploding them with inflammable air, I believe I may say in any proportion, is not at all acid; perhaps, because if they are mixed in such a proportion as that the burnt air is not much phlogisticated, the explosion is too weak, and not accompanied with sufficient heat.
All the foregoing experiments, on the explosion of inflammable air with common and dephlogisticated airs, except those which relate to the cause of the acid found in the water, were made in the summer of the year 1781, and were mentioned by me to Dr. PRIESTLEY, who in consequence of it made some experiments of the same kind, as he relates in a paper printed in the preceding volume of the Transactions. During the last summer also, a friend of mine gave some account of them to M. LAVOISIER, as well as of the conclusion drawn from them, that dephlogisticated air is only water deprived of phlogiston; but at that time so far was M. LAVOISIER from thinking any such opinion warranted, that, till he was prevailed upon to repeat the experiment himself, he found some difficulty in believing that nearly the whole of the two airs could be converted into water. It is remarkable, that neither of these gentlemen found any acid in the water produced by the combustion; which might proceed from the latter having burnt the two airs in a different manner from what I did; and from the former having used a different kind of inflammable air, namely, that from charcoal [i.e., carbon monoxide], and perhaps having used a greater proportion of it.
Before I enter into the cause of these phaenomena, it will be proper to take notice, that phlogisticated air appears to be nothing else than the nitrous acid united to phlogiston; for when nitre is deflagrated with charcoal, the acid is almost entirely converted into this kind of air. That the acid is entirely converted into air, appears from the common process for making what is called clyssus of nitre; for if the nitre and charcoal are dry, scarce any thing is found in the vessels prepared for condensing the fumes; but if they are moist a little liquor is collected, which is nothing but the water contained in the materials, impregnated with a little volatile alkali, proceeding in all probability from the imperfectly burnt charcoal, and a little fixed alkali, consisting of some of the alkalized nitre carried over by the heat and watery vapours. As far as I can perceive too, at present, the air into which much the greatest part of the acid is converted, differs in no respect from common air phlogisticated. A small part of the acid, however, is turned into nitrous air, and the whole is mixed with a good deal of fixed, and perhaps a little inflammable air, both proceeding from the charcoal.
It is well known, that the nitrous acid is also converted by phlogistication into nitrous air, in which respect there seems a considerable analogy between that and the vitriolic acid; for the vitriolic acid, when united to a smaller proportion of phlogiston, forms the volatile sulphureous acid and vitriolic acid air, both of which, by exposure to the atmosphere, lose their phlogiston, though not very fast, and are turned back into vitriolic acid; but, when united to a greater proportion of phlogiston, it forms sulphur, which shews no signs of acidity, unless a small degree of affinity to alkalies can be called so, and in which the phlogiston is more strongly adherent, so that it does not fly off when exposed to the air, unless assisted by a heat sufficient to set it on fire. In like manner the nitrous acid, united to a certain quantity of phlogiston, forms nitrous fumes and nitrous air, which readily quit their phlogiston to common air; but when united to a different, in all probability a larger quantity, it forms phlogisticated air, which shews no signs of acidity, and is still less disposed to part with its phlogiston than sulphur.
This being premised, there seem two ways by which the phaenomena of the acid found in the condensed liquor may be explained; first, by supposing that dephlogisticated air contains a little nitrous acid which enters into it as one of its component parts, and that this acid, when the inflammable air is in a sufficient proportion, unites to the phlogiston, and is turned into phlogisticated air, but does not when the inflammable air is in too small a proportion; and, secondly, by supposing that there is no nitrous acid mixed with, or entering into the composition of, dephlogisticared air, but that, when this air is in a sufficient proportion, part of the phlogisticated air with which it is debased is, by the strong affinity of phlogiston to dephlogisticated air, deprived of its phlogiston and turned into nitrous acid; whereas, when the dephlogisticated air is not more than sufficient to consume the inflammable air, none then remains to deprive the phlogisticated air of its phlogiston, and turn it into acid.
If the latter explanation be true, I think, we must allow that dephlogisticated air is in reality nothing but dephlogisticated water, or water deprived of its phlogiston; or, in other words, that water consists of dephlogisticated air united to phlogiston; and that inflammable air is either pure phlogiston, as Dr. PRIESTLEY and Mr. KIRWAN suppose, or else water united to phlogiston;11 since, according to this supposition, these two substances united together form pure water. On the other hand, if the first explanation be true, we must suppose that dephlogisticated air consists of water united to a little nitrous acid and deprived of its phlogiston; but still the nitrous acid in it must make only a very small part of the whole, as it is found, that the phlogisticated air, which it is converted into, is very small in comparison of the dephlogisticated air.
I think the second of these explanations seems much the most likely; as it was found, that the acid in the condensed liquor was of the nitrous kind, not only when the dephlogisticated air was prepared from red precipitate, but also when it was procured from plants or from turbith mineral: and it seems not likely, that air procured from plants, and still less likely that air procured from a solution of mercury in oil of vitriol, should contain any nitrous acid.
Another strong argument in favour of this opinion is, that dephlogisticated air yields no nitrous acid when phlogisticated by liver of sulphur: for if this air contains nitrous acid, and yields it when phlogisticated by explosion with inflammable air, it is very extraordinary that it should not do so when phlogisticated by other means.
But what forms a stronger and, I think, almost decisive argument in favour of this explanation is, that when the dephlogisticated air is very pure, the condensed liquor is made much more strongly acid by mixing the air to be exploded with a little phlogisticated air, as appears by the following experiments.
A mixture of 18,500 grain measures of inflammable air with 9750 of dephlogisticated air procured from red precipitate were exploded in the usual manner; after which, a mixture of the same quantities of the same dephlogisticated and inflammable air, with the addition of 2500 of air phlogisticated by iron filings and sulphur, was treated in the same manner. The condensed liquor, in both experiments, was acid, but that in the latter evidently more so, as appeared also by saturating each of them separately with marble powder, and precipitating the earth by fixed alkali, the precipitate of the second experiment weighing one-fifth of a grain, and that of the first being several times less. The standard of the burnt air in the first experiment was 1,86, and in the second only 0,9.
It must be observed, that all circumstances were the same in these two experiments, except that in the latter the air to be exploded was mixed with some phlogisticated air, and that in consequence the burnt air was more phlogisticated than in the former; and from what has been before said, it appears, that this latter circumstance ought rather to have made the condensed liquor less acid; and yet it was found to be much more so, which shews strongly that it was the phlogisticated air which furnished the acid.
As a further confirmation of this point, these two comparative experiments were repeated with a little variation, namely, in the first experiment there was first let into the globe 1500 of dephlogisticated air, and then the mixture, consisting of 12,200 of dephlogisticated air and 25,900 of inflammable, was let in at different times as usual. In the second experiment, besides the 1500 of dephlogisticated air first let in, there was also admitted 2500 of phlogisticated air, after which the mixture, consisting of the same quantities of dephlogisticated and inflammable air as before, was let in as usual. The condensed liquor of the second experiment was about three times as acid as that of the first, as it required 119 grains of a diluted solution of salt of tartar to saturate it, and the other only 37. The standard of the burnt air was 0,78 in the second experiment, and 1,96 in the first.
The intention of previously letting in some dephlogisticated air in the two last experiments was, that the condensed liquor was expected to become more acid thereby, as proved actually to be the case.
In the first of these two experiments, in order that the air to be exploded should be as free as possible from common air, the globe was first filled with a mixture of dephlogisticated and inflammable air, it was then exhausted, and the air to be exploded let in; by which means, though the globe was not perfectly exhausted, very little common air could be left in it. In the first set of experiments this circumstance was not attended to, and the purity of the dephlogisticated air was forgot to be examined in both sets.
From what has been said there seems the utmost reason to think, that dephlogisticated air is only water deprived of its phlogiston, and that inflammable air, as was before said, is either phlogisticated water, or else pure phlogiston; but in all probability the former. . . .
There are several memoirs of M. LAVOISIER published by the Academy of Sciences, in which he intirely discards phlogiston, and explains those phaenomena which have been usually attributed to the loss or attraction of that substance, by the absorption or expulsion of dephlogisticated air; and as not only the foregoing experiments, but most other phaenomena of nature, seem explicable as well, or nearly as well, upon this as upon the commonly believed principle of phlogiston, it may be proper briefly to mention in what manner I would explain them on this principle, and why I have adhered to the other. In doing this, I shall not conform strictly to his theory, but shall make such additions and alterations as seem to suit it best to the phaenomena; the more so, as the foregoing experiments may, perhaps, induce the author himself to think some such additions proper.
According to this hypothesis, we must suppose, that water consists of inflammable air united to dephlogisticated air; that nitrous air, vitriolic acid air, and the phosphoric acid, are also combinations of phlogisticated air, sulphur, and phosphorus, with dephlogisticated air; and that the two former, by a further addition of the same substance, are reduced to the common nitrous and vitriolic acids; that the metallic calces consist of the metals themselves united to the same substance, commonly, however, with a mixture of fixed air; that on exposing the calces of the perfect metals to a sufficient heat, all the dephlogisticated air is driven off, and the calces are restored to their metallic form; but as the calces of the imperfect metals are vitrified by heat, instead of recovering the metallic form, it should seem as if all the dephlogisticated air could not be driven off from them by heat alone. In like manner, according to this hypothesis, the rationale of the production of dephlogisticated air from red precipitate is, that during the solution of the quicksilver in the acid and the subsequent calcination, the acid is decompounded, and quits part of its dephlogisticated air to the quicksilver, whereby it comes over in the form of nitrous air, and leaves the quicksilver behind united to dephlogisticated air, which, by a further increase of heat, is driven off, while the quicksilver reassumes its metallic form. In procuring dephlogisticated air from nitre, the acid is also decompounded; but with this difference, that it suffers some of its dephlogisticated air to escape, while it remains united to the alkali itself, in the form of phlogisticated nitrous acid. As to the production of dephlogisticated air from plants, it may be said, that vegetable substances consist chiefly of various combinations of three different bases, one of which, when united to dephlogisticated air, forms water, another fixed air, and the third phlogisticated air; and that by means of vegetation each of these substances are decomposed, and yield their dephlogisticated air; and that in burning they again acquire dephlogisticated air, and are restored to their pristine form.
It seems, therefore, from what has been said, as if the phaenomena of nature might be explained very well on this principle, without the help of phlogiston; and indeed, as adding dephlogisticated air to a body comes to the same thing as depriving it of its phlogiston and adding water to it, and as there are, perhaps, no bodies entirely destitute of water, and as I know no way by which phlogiston can be transferred from one body to another, without leaving it uncertain whether water is not at the same time transferred, it will be very difficult to determine by experiment which of these opinions is the truest; but as the commonly received principle of phlogiston explains all phaenomena, at least as well as M. LAVOISIER’S, I have adhered to that. There is one circumstance also, which though it may appear to many not to have much force, I own has some weight with me; it is, that as plants seem to draw their nourishment almost intirely from water and fixed and phlogisticated air, and are restored back to those substances by burning, it seems reasonable to conclude, that notwithstanding their infinite variety they consist almost intirely of various combinations of water and fixed and phlogisticated air, united according to one of these opinions to phlogiston, and deprived according to the other of dephlogisticated air; so that, according to the latter opinion, the substance of a plant is less compounded than a mixture of those bodies into which it is resolved by burning; and it is more reasonable to look for great variety in the more compound than in the more simple substance.
Another thing which M. LAVOISIER endeavours to prove is, that dephlogisticated air is the acidifying principle. From what has been explained it appears, that this is no more than saying, that acids lose their acidity by uniting to phlogiston, which with regard to the nitrous, vitriolic, phosphoric, and arsenical acids is certainly true. The same thing, I believe, may be said of the acid of sugar; and Mr. LAVOISIER’s experiment is a strong confirmation of BERGMAN’s opinion, that none of the spirit of nitre enters into the composition of the acid, but that it only serves to deprive the sugar of part of the phlogiston. But as to the marine acid and acid of tartar, it does not appear that they are capable of losing their acidity by any union with phlogiston. It is to be remarked also, that the acids of sugar and tartar, and in all probability almost all the vegetable and animal acids, are by burning reduced to fixed and phlogistieated air, and water, and therefore contain more phlogiston, or less dephlogisticared air, than those three substances.
1 W. F. Magie, "A Source Book in Physics," New York, 1935, pp. 105–111; K. F. Mather and S. L. Mason, "A Source Book in Geology," New York, 1939, pp. 103–107.
"Electrical Researches, (of Henry Cavendish), written between 1771 and 1781, ed. from the Original MSS. by James Clerk Maxwell," Cambridge, 1879.
2 These, together with a number of unpublished manuscripts, have been edited by Edward Thorpe, "The Scientific Papers of the Honourable Henry Cavendish, F.R.S.," Cambridge, 1921, 2 vols.
3Philosophical Transactions,56: 141–184 (1766); Thorpe, op. cit., vol. 2, pp. 77–101.
4 Sulphur is allowed by chymists, to consist of the plain vitriolic acid united to phlogiston. The volatile sulphureous acid appears to consist of the same acid united to a less proportion of phlogiston than what is required to form sulphur. A circumstance which I think shews the truth of this, is that if oil of vitriol be distilled, from sulphur, the liquor, which comes over, will be the volatile sulphureous acid.
5 As the quantity of lute used was but small, and as this kind of lute does not lose a great deal of its weight by being kept in a moderately dry room, no sensible error could arise from the drying of the lute during the experiment.
6 J. R. Partington, "The Composition of Water," London, 1928; G. Wilson, "The Life of the Honourable Henry Cavendish," London, 1851; J. P. Muirhead, "Correspondence of the Late James Watt," London, 1846. The papers of Cavendish on the composition of water are reprinted in full in the Alembic Club Reprints, No. 3, Edinburgh, 1899.
7 S. M. Edelstein, Chymia,1: 123–127 (1948).
8Philosophical Transactions,74: 119–153 (1784); also found in Thorpe, op. cit., vol. 2, pp. 161–181; and in Alembic Club Reprints, No. 3, "Experiments on Air. Papers published in the Philosophical Transactions by the Hon. Henry Cavendish, F.R.S. (1784–1785)," Edinburgh, 1893.
9 Dr. PRIESTLEY, I am informed, has since found the experiment not to succeed.
10 In order to prevent any water from getting into this tube, while dipped under water to let it up into the glass jar, a bit of wax was stuck upon the end of it, which was rubbed off when raised above the surface of the water.
11 Either of these suppositions will agree equally well with the following experiments; but the latter seems to me much the most likely. What principally makes me think so is, that common or dephlogisticated air do not absorb phlogiston from inflammable air, unless assisted by a red heat, whereas they absorb the phlogiston of nitrous air, liver of sulphur [potassium polysulfide], and many other substances, without that assistance; and it seems inexplicable, that they should refuse to unite to pure phlogiston, when they axe able to extract it from substances to which it has an affinity; that is, that they should overcome the affinity of phlogiston to other substances, and extract it from them, when they will not even unite to it when presented to them. On the other hand, I know no experiment which shews inflammable air to be pure phlogiston rather than an union of it with water, unless it be Dr. PRIESTLEY’s experiment of expelling inflammable air from iron by heat alone. I am not sufficiently acquainted with the circumstances of that experiment to argue with certainty about it; but I think it much more likely, that the inflammable air was formed by the union of the phlogiston of the iron filings with the water dispersed among them, or contained in the retort or other vessel in which it was heated; and in all probability this was the cause of the separation of the phlogiston, as iron seems not disposed to part with its phlogiston by heat alone, without being assisted by the air or some other substance.