|
|
A Source Book in Astronomy and Astrophysics, 1900-1975
Contents:
Show Summary
Hide Summary
General SummaryThe theory of the evolution of species implies that we can extrapolate backward in time to the point when the first living organism was formed. In the 1930s Aleksandr Oparin opened the argument that the first organism could have been generated spontaneously if sufficient quantities of organic compounds were present in the shallow seas of the primitive earth. Under this assumption, a key problem in explaining the origin of life becomes the one of determining how the organic constituents of living matter arose in the first place. Many astronomers believe that the primitive atmosphere of the earth was rich in hydrogen, quite unlike the present oxygen-rich atmosphere. Harold Urey has persuasively argued that the cold, original atmosphere of the earth must have been composed of the stable molecules of methane, ammonia, water, and hydrogen. This view is partly a result of the old observation that Jupiter, Saturn, and Titan contain ammonia and methane and partly a result of the fact that the most abundant element of the primitive solar nebula must have been hydrogen. In the paper given here, Stanley Miller describes how an electrical discharge in a simulation of the primitive earth atmosphere can give rise to one of the basic building blocks of living organisms, the amino acids. Since that time, a number of experiments have been performed in which the simple molecules of the earth’s primitive atmosphere are converted into more complex molecules by the action of electric discharge, ultraviolet light, and ionizing radiation. As a result of these experiments, we believe that the carbon and hydrogen in methane, the hydrogen and oxygen in water, and the hydrogen and nitrogen in ammonia, can be liberated by various radiation processes and can combine to produce all the complex molecules that subsequently form the living species. Recently, an alternative possibility has been introduced, according to which organic compounds are formed in the solar nebula by reactions involving no external energy sources. Straight-chained hydrocarbons (thought by many to be indigenous components of meteorites) can be produced in the terrestrial laboratory by the Fischer-Tropsch reaction, in which carbon monoxide mixes with molecular hydrogen in the presence of an iron catalyst to form both water and hydrocarbons. In addition, when ammonia is added to the gas mixture, intermediates are generated that lead to amino acids and other organic compounds that have been found in meteorites. These developments point to the possibility that some of the basic components of biological life could have been formed through the interaction of abundant organic molecules in the primitive solar nebula or on the primitive earth. Nevertheless, special physical conditions are required for the Fischer-Tropsch reaction, and it now appears that a variety of reactions, including those involving electrical discharges and an aqueous environment, are needed to account for all the organic compounds synthesized on the primitive earth.
A Production of Amino Acids under Possible Primitive Earth Conditions
Stanley L. Miller
(Science 117, 528–529 [1953])
THE IDEA that the organic compounds that serve as the basis of life were formed when the earth had an atmosphere of methane, ammonia, water, and hydrogen instead of carbon dioxide, nitrogen, oxygen, and water was suggested by Oparin1 and has been given emphasis recently by Urey2 and Bernal.3
In order to test this hypothesis, an apparatus was built to circulate
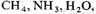
and
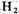
past an electric discharge. The resulting mixture has been tested for amino acids by paper chromatography. Electrical discharge was used to form free radicals instead of ultraviolet light, because quartz absorbs wavelengths short enough to cause photo-dissociation of the gases. Electrical discharge may have played a significant role in the formation of compounds in the primitive atmosphere.
Water is boiled in one flask, mixes with the gases in another flask, circulates past the electrodes, condenses and empties back into the boiling flask. The U-tube prevents circulation in the opposite direction. The acids and amino acids formed in the discharge, not being volatile, accumulate in the water phase. The circulation of the gases is quite slow, but this seems to be an asset, because production was less in a different apparatus with an aspirator arrangement to promote circulation. The discharge, a small corona, was provided by an induction coil designed for detection of leaks in vacuum apparatus.
The experimental procedure was to seal off the opening in the boiling flask after adding 200 ml of water, evacuate the air, add 10 cm pressure of
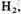
20 cm of
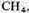
and 20 cm of
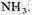
The water in the flask was boiled, and the discharge was run continuously for a week.
During the run the water in the flask became noticeably pink after the first day, and by the end of the week the solution was deep red and turbid. Most of the turbidity was due to colloidal silica from the glass. The red color is due to organic compounds adsorbed on the silica. Also present are yellow organic compounds, of which only a small fraction can be extracted with ether, and which form a continuous streak tapering off at the bottom on a one-dimensional chromatogram run in butanol-acetic acid. These substances are being investigated further.
At the end of the run the solution in the boiling flask was removed and 1 ml of saturated
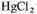
was added to prevent the growth of living organisms. The ampholytes were separated from the rest of the constituents by adding
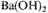
and evaporating in vacuo to remove amines, adding
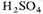
and evaporating to remove the acids, neutralizing with
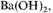
filtering and concentrating in vacuo.
The amino acids are not due to living organisms because their growth would be prevented by the boiling water during the run, and by the
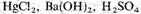
during the analysis.
In figure 33.1 is shown a paper chromatogram run in n-butanol-acetic acid-water mixture followed by water-saturated phenol, and spraying with ninhydrin. Identification of an amino acid was made when the Rf value (the ratio of the distance traveled by the amino acid to the distance traveled by the solvent front), the shape, and the color of the spot were
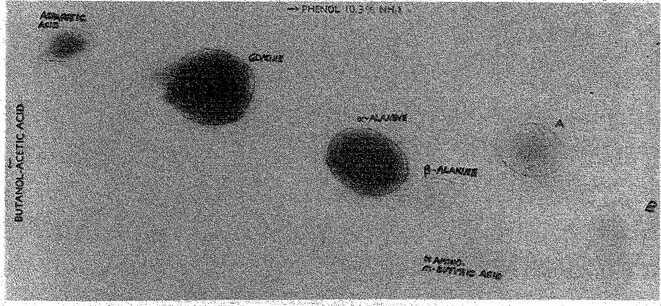 Fig. 33.1 A paper chromatogram showing the presence of amino acids in a mixture of methane, ammonia, water, and hydrogen that has been subjected to an electrical discharge. (Courtesy Stanley Miller.) Fig. 33.1 A paper chromatogram showing the presence of amino acids in a mixture of methane, ammonia, water, and hydrogen that has been subjected to an electrical discharge. (Courtesy Stanley Miller.)
the same on a known, unknown, and mixture of the known and unknown; and when consistent results were obtained with chromatograms using phenol and 77% ethanol.
On this basis glycine, α-alanine and β-alanine are identified. The identification of the aspartic acid and α-amino-n-butyric acid is less certain because the spots are quite weak. The spots marked A and B are unidentified as yet, but may be beta and gamma amino acids. These are the main amino acids present, and others are undoubtedly present but in smaller amounts. It is estimated that the total yield of amino acids was in the milligram range.
In this apparatus an attempt was made to duplicate a primitive atmosphere of the earth, and not to obtain the optimum conditions for the formation of amino acids. Although in this case the total yield was small for the energy expended, it is possible that, with more efficient apparatus (such as mixing of the free radicals in a flow system, use of higher hydrocarbons from natural gas or petroleum, carbon dioxide, etc., and optimum ratios of gases), this type of process would be a way of commercially producing amino acids.
1. A. I. Oparin, The Origin of Life (New York: Macmillan Co., 1938; 3rd ed., New York: Academic Press, 1957).
2. H. C. Urey, The Planets: Their Origin and Development (New Haven: Yale University Press, 1952).
3. S. L. Miller and H. C. Urey, Science 130, 245 (1959); S. L. Miller and L. E. Orgel, The Origins of Life on Earth (Englewood Cliffs, N. J.: Prentice Hall, 1974).
4. S. L. Miller, H. C. Urey, and J. Oro, Journal of Molecular Evolution 9, 59 (1976).
1. A. I. Oparin, The Origin of Life (New York: Macmillan Co., 1938).
2. H. C. Urey, Proc. Natl. Acad. Sci. U.S. 38, 351 (1952); The Planets (New Haven: Yale University Press, 1952), chap. 4.
3. J. D. Bernal, Proc. Phys. Soc. (London) 62A, 537 (1949), 62B, 597 (1949); Physical Basis of Life (London: Routledge and Kegan Paul, 1951).
Contents:
Chicago:
Stanley L. Miller, "A Production of Amino Acids Under Possible Primitive Earth Conditions," A Source Book in Astronomy and Astrophysics, 1900-1975 in A Source Book in Astronomy and Astrophysics, 1900-1975, ed. Kenneth R. Lang and Owen Gingerich (Cambridge: Harvard University Press, 1979), 203–206. Original Sources, accessed January 5, 2026, http://www.originalsources.com/Document.aspx?DocID=4X9Y29PPHTQZGZK.
MLA:
Miller, Stanley L. "A Production of Amino Acids Under Possible Primitive Earth Conditions." A Source Book in Astronomy and Astrophysics, 1900-1975, Vol. 117, in A Source Book in Astronomy and Astrophysics, 1900-1975, edited by Kenneth R. Lang and Owen Gingerich, Cambridge, Harvard University Press, 1979, pp. 203–206. Original Sources. 5 Jan. 2026. http://www.originalsources.com/Document.aspx?DocID=4X9Y29PPHTQZGZK.
Harvard:
Miller, SL, 'A Production of Amino Acids Under Possible Primitive Earth Conditions' in A Source Book in Astronomy and Astrophysics, 1900-1975. cited in 1979, A Source Book in Astronomy and Astrophysics, 1900-1975, ed. , Harvard University Press, Cambridge, pp.203–206. Original Sources, retrieved 5 January 2026, from http://www.originalsources.com/Document.aspx?DocID=4X9Y29PPHTQZGZK.
|